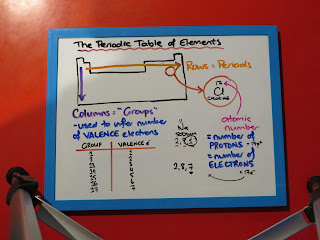
The Periodic Table of Elements is a fantastic tools for Chemists. If we know how to use it, we do not have to remember quite so many minor details - we can work them out from the Periodic Table instead!
The Columns (Groups) tell us the number of valence (outermost) electrons. These are the electrons that give each element its chemical reactions and properties, so that is a very useful thing to be able to work out.
The Rows (Periods) tell us how many electron shells an atom has its electrons in. That will be more useful in Year 13 and beyond. However, it makes for a good "check" that you have written electron arrangements correctly.
The relative atomic mass number tells us if an element has isotopes or not, but does not help chemists a lot with the reactions and properties of an element. We do use this value to do a lot of calculations in Chemistry, so know where to find on a Periodic Table.

Comments
Post a Comment